Teburin sinadarai wani tsarin kimiyya ne da ake amfani da shi wajen tsarawa da nuna dukkan sinadarai da ma’adanan da aka sani a duniya bisa tsarin iliminsu da siffofinsu. An ƙirƙiro shi ne don taimaka wa masana kimiyya su fahimci dangantaka tsakanin sinadarai da kuma sauƙaƙe karatu da bincike a fannin ilimin sinadarai wato (Chemistry). Wannan tebur yana da matuƙar muhimmanci a dukkan matakan ilimin kimiyya.
Teburin sinadarai yana daga cikin mafi muhimmancin ƙirƙire-ƙirƙire a fannin ilimin kimiyya. Ba wai kawai yana tsara bayanai ba ne, har ma yana bayar da haske game da suffofi, aikace-aikace, da dangantaka tsakanin sinadarai. Ilimi game da teburin yana taimakawa wajen fahimtar yadda duniya da jikin ɗan’adam ke aiki, wanda hakan ke haifar da ci gaba a fannoni daban-daban na rayuwa.
Tarihin teburin sinadarai
An fara ƙirƙirar jadawalin teburin sinadarai ne a ƙarshen ƙarni na 19, inda wani masanin kimiyya ɗan ƙasar Rasha mai suna Dmitri Mendeleev ya wallafa tsarin farko na teburin sinadarai a shekarar 1869. Mendeleev ya tsara sinadarai bisa nauyinsu da siffofins, inda ya bar wasu gurabe da yake da yaƙinin za a gano sabbin sinadarai a nan gaba. Wannan hasashe nasa ya zama gaskiya yayin da wasu sinadarai da bai riga ya gani ba daga baya aka gano su kuma suka dace da guraben da ya bari.
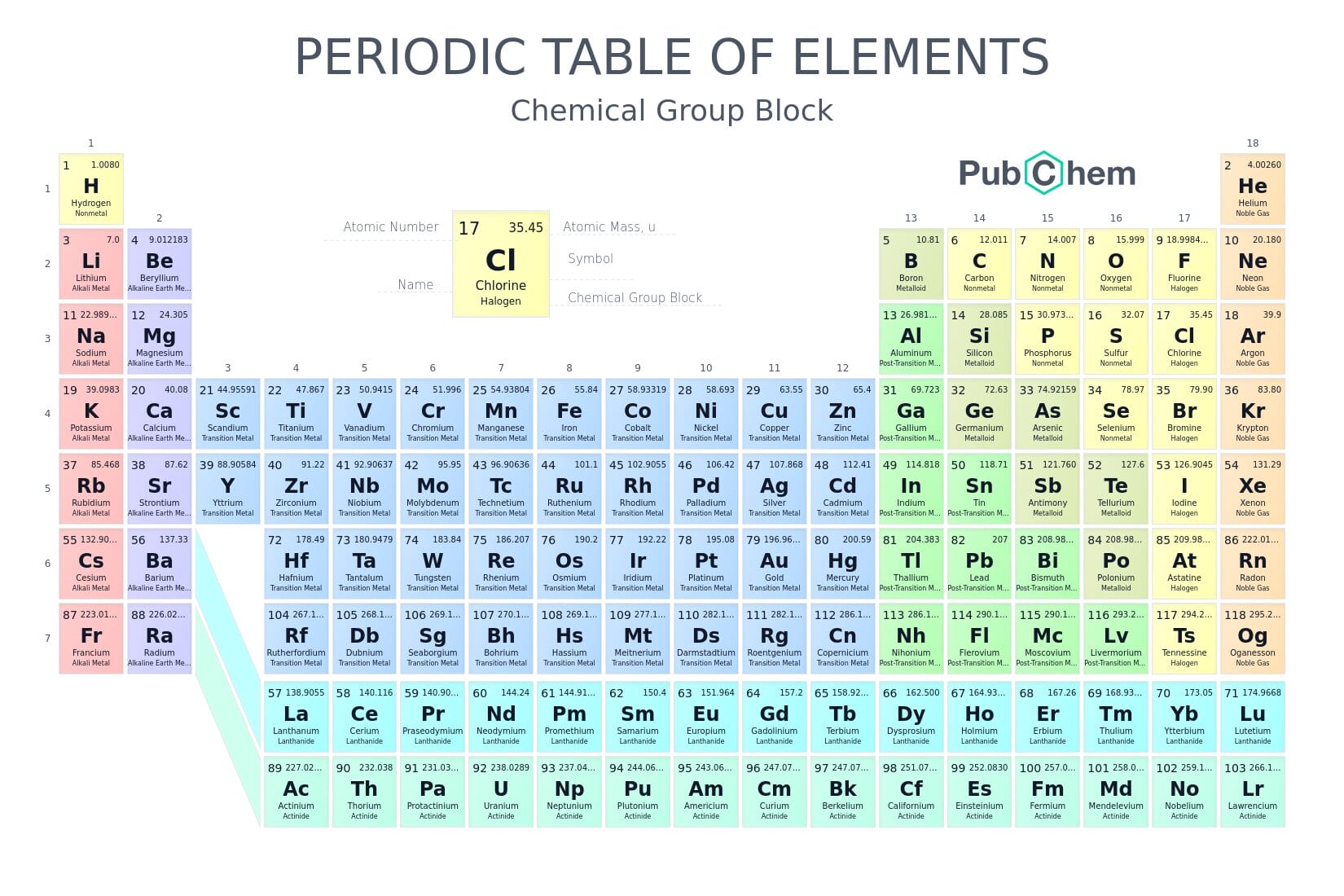
Teburin sinadarai wani babban ginshiƙi ne na ilimin kimiyya wanda ke bayyana ilimin da duniya ke da shi game da abubuwan da ke ƙunshe cikin sunadarai. Duk da cewa ya fara ne da sinadarai kaɗan, yau an kai matakin gano sinadarai har sama da dari. Yana taimakawa wajen haɗa sabbin sinadarai, gano ingantattun hanyoyin samar da makamashi, magunguna, kayan aiki, da inganta rayuwar bil’adama gaba ɗaya.
Tsarin teburin sinadarai
Teburin sinadarai yana da ginshiƙai biyu masu mahimmanci:
- Layuka (Periods) – Waɗanda ke shimfiɗe a kwance daga hagu zuwa dama. Akwai layuka guda 7 a bisa teburin.
- Rukunai (Groups) – Waɗanda ke tsaye daga sama zuwa ƙasa. Akwai har guda 18.
Sinadarai suna shiga teburin ne bisa tsarin lambar sinadarai (atomic number), wato yawan proton da ke cikin ƙwayar sinadari. An fara jera sinadarai a teburin ne da sinadarin Hydrogen (H) wanda ke da lamba ta 1, sannan aka ci gaba har zuwa sinadari mai lamba ta 118 wato Oganesson (Og).
Rabe-raben sinadarai a teburin
Sinadarai a cikin wannan jadawalin teburin suna da rabe-rabe kamar haka:
- Karafa(Metals): Su ne sinadarai da ma’adanan da suke da siffofi kamar ɗaukar zafi, ɗaukar wuta, da kuma sarrafuwa. Misalansu sun haɗa da: Iron (Fe), Copper (Cu), da Gold (Au).
- Wadanda ba ƙarafa ba (Non-metals): Waɗannan rukunin sinadarai ba su da siffofin ƙarafa. Misalansu sun haɗa: Oxygen (O), Nitrogen (N), da Chlorine (Cl).
- Na kusa da ƙarafa (Metalloids): Su ne sinadarai waɗanda ke da siffofi tsakanin sinadaran ƙarafa da waɗanda ba karafa ba. Misalansu sun haɗa da: Silicon (Si), Arsenic (As).
Manyan rukunan teburin sinadarai
- Rukunin Alkaline (Group 1): Waɗannan suna da matuƙar saurin shiga cikin wasu sinadaran. Misali: Sodium (Na), Potassium (K).
- Rukunin alkaline earth metals (Group 2): Wannan rukuni suna da ƙarfi amma ba kamar group 1 ba.
- Transition metals (Groups 3-12): Waɗanda ke tsakiyar teburin kuma suna da matuƙar muhimmanci a masana’antu. Misali: Iron, Copper, Zinc.
- Halogens (Group 17): Sinadarai ne masu saurin haɗuwa da wasu wanda hakan kan samar da sinadarin gishiri. Misali: Fluorine (F), Chlorine (Cl).
- Noble Gases (Group 18): Sinadarai masu cikakken tsarin electrons, don haka ba su da sauƙin shiga cikin sinadaran. Misali: Helium (He), Neon (Ne), Argon (Ar).
Lantanides da Actinides
Waɗannan rukuni guda biyu suna ƙasa a cikin teburin:
Lantanides
Sun haɗa da sinadarai daga Lantanum (La) zuwa Lutetium (Lu), suna da amfani a fasahar zamani kamar wajen sarrafa fuskokin talabijin da wayoyi.
Actinides
Sun haɗa da sinadarai masu nauyin gaske kamar Uranium (U) da Plutonium (Pu), wadanda ke da amfani wajen sarrafa makamashin nukiliya.
Muhimmancin teburin sinadarai
Teburin sinadarai ba kawai ginshiki ne na kimiyya ba, har ila yau yana nuna yadda ilimi ke tafiya cikin tsari, kuma yana ƙarfafa bincike, haɗin gwiwa, da ci gaba. A fannin karatun kimiyya da bincike, teburin sinadarai yana taka muhimmiyar rawa:
- Sauƙaƙa Ilimi: Teburin na taimakawa ɗalibai da masana su fahimci dangantaka tsakanin sinadarai cikin sauƙi.
- Ƙirƙirar sabbin sunadarai: Yana taimakawa wajen gano sabbin sinadarai ko ƙirƙirar sababbin sinadarai ta hanyar hada su.
- Magunguna: Masana kimiyya suna amfani da teburin wajen zaɓar sinadarai da za su iya yin tasiri a jikin ɗan’adam.
- Samar da kayayyaki: Ana amfani da sinadarai sosai wajen samar da kayayyakin amfani kamar batir, wayoyin hannu, fitilu, da dai sauran su.
- Ilmin halittu da sinadarai (Biochemistry): Teburin yana taimakawa wajen fahimtar yadda jikin mutum ke amfani da sinadarai kamar su oxygen, carbon, calcium da sauransu.
- Abinci: Ana amfani da teburin wajen gano sinadarai masu gina jiki da kuma masu illa.
- Masana’antu: Teburin na da matuƙar amfani wajen zaɓin sinadarai don amfani a fannoni kamar magunguna, abinci, noma, da makamashi.
Sabbin sinadaran da aka gano
A shekarun baya-bayan nan, an ƙara wasu sabbin sinadarai a kan teburin, kamar su Nihonium (Nh), Moscovium (Mc), Tennessine (Ts) da Oganesson (Og). Duk da cewa ba su da yawa a doron ƙasa, an gano su ne a ɗakin gwaji ta hanyar haɗa wasu sinadaran.
Abin sha’awa game da teburin
Ɗaya daga cikin abin ban mamaki da sha’awa game da teburin sinadarai shi ne yadda yake nuna tsari da yanayin sinadarai. Akwai ƙa’idar yanayin lokaci (Periodic Law) wacce ke nuna cewa siffofin sinadarai na maimaituwa bisa lamba atomik.
Yadda teburin sinadarai ke aiki
Teburin sinadarai ya kasance kamar taswira ce ta dukkan sinadaran da Allah ya halitta ko kuma aka ƙirƙira. An tsara sinadarai a cikin wannan tebur ne bisa ga:
- Lambar Atomik (Atomic Number): Wannan na nuna adadin yawan protons da ke cikin kwayar sinadari. Wannan lamba tana da muhimmanci domin tana bambanta kowanne sinadari da wani.
- Tsarin electrons: Yadda electrons suke zagaye ƙwayar sinadari yana taimakawa wajen fahimtar siffofin sinadarin; wato ko yana da sauƙin shiga cikin wasu sinadarai ko a’a.
- Suffofi (physical and chemical properties): Irin su launi, narkewa, tafasa, taushi ko tauri da dai sauransu.
A tsari na wannan jadawalin teburi, ana amfani da waɗannan abubuwa da aka ambata wajen tsara sinadarai a cikin rukuni-rukuni da ke bayyana irin siffofinsu da kuma alaƙarsu da juna.
Bayanin launukan
A teburin sinadarai na zamani, ana amfani da launuka don bayyana rukunai daban-daban, waɗannan launuka sun haɗa da:
- Launin ja ko ruwan kasa: Ana amfani da wannan launi ne don nuna sinadaran da ke a matsayin ƙarafa.
- Launin shudi ko kore: Shi ma wannan launi ana amfani da shi ne don nuna sinadaran da ba ƙarafa ba ne a cikin wannan jadawalin teburin na sunadarai.
- Launin ruwan hoda: Shi kuwa wannan launi na ruwan hoda ana amfani da shi ne wajen bayyana sinadaran da ke kusa da ƙarafa.
- Launin rawaya: Launin rawaya a cikin jadawalin teburin sunadarai ana amfani da shi ne domin bayyana sinadarai da ke a matsayin iska (gases).
- Launin ruwan toka: A ƙarshe kuma wannan launi na ruwan riƙa na wakiltar sinadaran da ba a gano sosai ba.
Wannan tsari yana taimaka wa ɗalibai da masana kimiyya su gane tare da fahimtar dangantakar sinadarai da matsayinsu.
Tasirin teburin ga rayuwar yau da kullum
Teburin ba wai kawai ɓangaren ilimin kimiyya ba ne, har ila yau yana da amfani a rayuwar yau da kullum, misali:
- Iron (Fe): Ana amfani da shi wajen gina gine-gine, motoci, da kayan aiki.
- Copper (Cu): Ana amfani da shi wajen wutar lantarki da na’urorin sadarwa.
- Aluminum (Al): Yana da sauƙin nauyi, ana amfani da shi wajen yin tukwane, kwalaye, da jikin jirgi.
- Carbon (C): Na daga cikin ginshiƙin sinadarai, kuma yana da nau’i biyu masu ban sha’awa: graphite da diamond.
- Calcium (Ca): Yana tallafa wa ƙashi da haƙora, yana kuma taimakawa wajen motsin tsoka da jijiyoyi.
Yadda za a fahimtar teburin
Ga yadda za a iya fassara bayani daga alamomin sinadarai:
- Alamar sinadari (Chemical symbol): Misali, “O” na nufin Oxygen.
- Lamba atomik (Atomic number): Misali, Oxygen yana da lamba ta 8 – ke nan yana da adadin protons guda 8 kamar yadda aka bayyana.
- Nauyin atom (Atomic mass): Wannan yana nuna nauyin ƙwayar zarra ta sinadarin, misali 15.999 ga sinadarin oxygen.
- Sunan sinadari: Ana rubuta shi cikakke, misali: Hydrogen ko Magnesium.
Mutanen da aka karrama dalilin hidimta wa teburin
Dmitri Mendeleev
Shi wanda ya fara ƙirƙirar jadawalin teburin kuma an amince da shi a matsayin wanda ya assasa wannan muhimmin sashe na ilimin kimiyya.
Glenn T. Seaborg
Ya taka rawa wajen gano sinadarai masu nauyi kamar Plutonium da kuma tsarin na zamani na lanthanides da actinides. A dalilin rawar da suka taka, wasu sinadarai ma aka sa musu sunansu don girmamawa, kamar sinadarin Seaborgium (Sg).
Manazarta
Pauling, C, L., Lagowski, & J.J. (2025, June 27). Periodic table | Definition, Elements, Groups, Charges, Trends, & Facts. Encyclopedia Britannica.
Ptable (n.d.). Periodic Table Ptable
International Union of Pure and Applied Chemistry. (2022, September 12). Periodic Table of Elements – IUPAC | International Union of Pure and Applied Chemistry.
PubChem. (n.d.). Periodic Table of elements. PubChem.
*****
Duk maƙalun da kuka karanta a wannan taska ta Bakandamiya, marubuta da editocinmu ne suka rubuta. Kuma kowace maƙala da aka buga ta bi muhimman matakai na tantancewa don ganin cewa bayanan dake cikinta sun inganta.
Idan kuma an ga wani kuskure a cikin kowace maƙalarmu, a sanar da mu. Za mu yi bincike sannan mu gyara gwargwadon fahimtarmu.
